Emission energy of azole-based ionic iridium(iii) complexes: a theoretical study†
Abstract
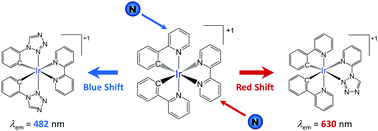
A theoretical density functional theory study has been performed on different families of cationic cyclometallated Ir(III) complexes with the general formula [Ir(C^N)2(N^N)]+ and azole-based ligands. The goal was to investigate the effect that the number and position of the nitrogen atoms of the azole ring have on the electronic structure and emission wavelength of the complex. The increase in the number of nitrogen atoms changes the relative energy of the HOMO and LUMO levels and leads to a gradual shift in the emission wavelength that can be larger than 100 nm. The direction of the shift however depends on the ligand in which the azole ring is introduced. The emission shifts to bluer wavelengths when the azole forms part of the cyclometallating C^N ligands, whereas it shifts to the red when the 5-membered ring is incorporated into the ancillary N^N ligand. The position of the nitrogen atoms in the azole ring also plays an important role in determining the emission energy. Complexes with phenyl-azole C^N ligands bearing a nitrogen in the azole position to which the phenyl is linked show a markedly blue-shifted emission compared to complexes with the same number of nitrogen atoms in the azole ring and bearing a carbon atom in that position. Therefore, when comparing the emission properties of azole-based [Ir(C^N)2(N^N)]+ complexes, not only the number of nitrogen atoms of the azole but also their position in the ring and the ligand where the azole ring is incorporated should be taken into account.
- This article is part of the themed collection: Luminescent Complexes and Materials for Light-Emitting Devices

 Please wait while we load your content...
Please wait while we load your content...