Cationic, luminescent cyclometalated iridium(iii) complexes based on substituted 2-phenylthiazole ligands†
Abstract
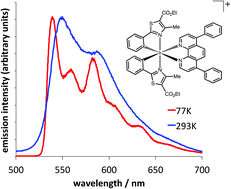
Ten cationic heteroleptic iridium(III) complexes, [Ir(emptz)2(N^N)](PF6) were prepared from a cyclometalated iridium bridged-chloride dimer involving two ethyl-4-methylphenylthiazole-5-carboxylate (emptz) ligands. One X-ray crystallographic study was undertaken where the ancillary N^N ligand was 4,7-diphenyl-1,10-phenanthroline and revealed the anticipated structure, showing a distorted octahedral coordination geometry at Ir(III). The complexes were visibly luminescent with modestly structured emission at 540–590 nm and lifetimes (60–340 ns) consistent with phosphorescence. TD-DFT calculations suggest that strong MLCT character contributes to the visible absorption characteristics, whilst the moderately structured emission profiles indicate a 3MLCT/3IL admixture of states to the phosphorescence.
- This article is part of the themed collection: Luminescent Complexes and Materials for Light-Emitting Devices


 Please wait while we load your content...
Please wait while we load your content...