Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures†
Abstract
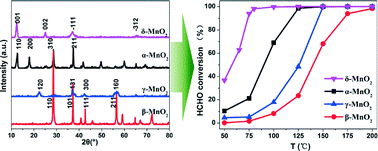
α-, β-, γ- and δ-MnO2 catalysts were prepared by a hydrothermal method and tested for the catalytic oxidation of formaldehyde (HCHO) at low temperature. Dramatic differences in activities among the MnO2 catalysts with different crystal structures were observed. The δ-MnO2 catalyst exhibited the best activity among the four catalysts and achieved nearly complete HCHO conversion at 80 °C, while the α-, β- and γ-type MnO2 obtained 100% HCHO conversion at 125 °C, 200 °C, 150 °C, respectively. The catalysts were next characterized by Brunauer–Emmett–Teller (BET), X-ray diffraction (XRD), Field-Emission Scanning Electron Microscopy (FE-SEM), temperature-programmed reduction by H2 (H2-TPR), X-ray photoelectron spectroscopy (XPS) and temperature-programmed desorption of HCHO (HCHO-TPD) methods to investigate the factors influencing the catalytic activity. Based on the characterization results, it is supposed that the tunnel structure and active lattice oxygen species are the main factors that contribute to the excellent performance of δ-MnO2. According to the high catalytic performance and facile preparation process, δ-MnO2 may potentially be used as a support in applications of supported catalysts.

 Please wait while we load your content...
Please wait while we load your content...