Quaternary ammonium hydroxide as a metal-free and halogen-free catalyst for the synthesis of cyclic carbonates from epoxides and carbon dioxide†
Abstract
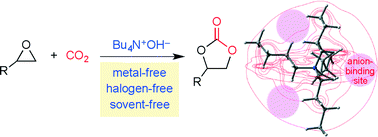
Tetrabutylammonium hydroxide (TBAH) and other quaternary ammonium hydroxides catalyzed the cycloaddition of CO2 to epoxides under solvent-free conditions to give cyclic carbonates. When TBAH was exposed to CO2, TBAH was converted into tetrabutylammonium bicarbonate (TBABC), which was a catalytically active species. A D-labeled epoxide and an optically active epoxide were used to study the reaction mechanism, which invoked three plausible pathways. Among them, path A seemed to be predominant; the bicarbonate ion of TBABC attacks the less hindered C atom of the epoxide to generate a ring-opened alkoxide intermediate, which adds to CO2 to give a carbonate ion, and the subsequent cyclization yields a cyclic carbonate. Density functional theory (DFT) calculations successfully delineated the potential energy profile for each reaction pathway, among which path A was the lowest-energy pathway in accordance with the experimental results. The tetrabutylammonium (TBA) cation carries the positive charges on the H atoms, but not on the central N atom, and the positively charged H atoms close to the central N atom form an anion-binding site capable of stabilizing various anionic transition states and intermediates.

 Please wait while we load your content...
Please wait while we load your content...