Tellurium: a maverick among the chalcogens†
Abstract
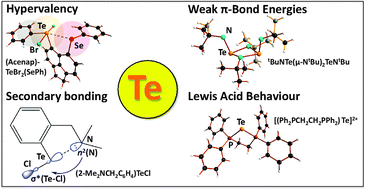
The scant attention paid to tellurium in both inorganic and organic chemistry textbooks may reflect, in part, the very low natural abundance of the element. Such treatments commonly imply that the structures and reactivities of tellurium compounds can be extrapolated from the behaviour of their lighter chalcogen analogues (sulfur and selenium). In fact, recent findings and well-established observations clearly illustrate that this assumption is not valid. The emerging importance of the unique properties of tellurium compounds is apparent from the variety of their known and potential applications in both inorganic and organic chemistry, as well as materials science. With reference to selected contemporary examples, this Tutorial Review examines the fundamental concepts that are essential for an understanding of the unique features of tellurium chemistry with an emphasis on hypervalency, three-centre bonding, secondary bonding interactions, σ and π-bond energies (multiply bonded compounds), and Lewis acid behaviour.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST

 Please wait while we load your content...
Please wait while we load your content...