Luminescence turn-on/off sensing of biological iron by carbon dots in transferrin†
Abstract
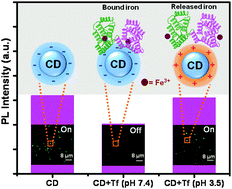
Iron is a key nutrient as well as a potential toxin for almost all living organisms. In mammalian cells, serum transferrin (Tf) is responsible for iron transport and its iron overload/deficiency causes various diseases. Therefore, closely regulated iron homeostasis is extremely essential for cellular metabolism. In the present article we report the pH-dependent luminescence turn-on/off sensing of bound Fe3+ ions of serum Tf by carbon dots (CDs) with the help of photoluminescence (PL) spectroscopy, FTIR spectroscopy, dynamic light scattering (DLS), circular dichroism (CD) and PL imaging techniques. At physiological pH (7.4), the intrinsic luminescence of CDs gets quenched in the presence of Tf as a consequence of ground-state association, which is driven by favorable electrostatic interactions between negatively charged CDs (−25.45 ± 1.23 mV) and positively charged Fe3+ ions of Tf. The estimated detection limit of Tf by CDs at physiological pH is found to be 1.82 μM (signal-to-noise ratio of 3), which is much lower than the in vivo plasma concentration of Tf (∼25–35 μM). Various thermodynamic parameters have been evaluated by using the van't Hoff equation. Importantly, the secondary structure of Tf remains unaltered upon association with CDs. However, at pH 3.5, no such luminescence quenching of CDs has been observed in the presence of Tf due to the lack of ground-state interactions between positively charged (+17.63 ± 0.84 mV) CDs and Tf. Furthermore, the results from UV-Vis and far-UV CD measurements revealed a significant conformational change of Tf at pH 3.5 relative to pH 7.4, which triggers the subsequent release of bound iron from Tf. PL microscopy of individual CD revealed significant luminescence quenching at the single particle level, which further supports the non-emissive ground-state complexation at pH 7.4. Our present results show that these chemically synthesized water-dispersed CDs have the ability to selectively sense the bound iron from released iron of Tf without any conformational perturbation and hence they can be used as potential biological iron sensors as well as luminescent markers for the detection of iron deficiency/overload in biological macromolecules.

 Please wait while we load your content...
Please wait while we load your content...