Functional ionic liquids for enhancement of Li-ion transfer: the effect of cation structure on the charge–discharge performance of the Li4Ti5O12 electrode†
Abstract
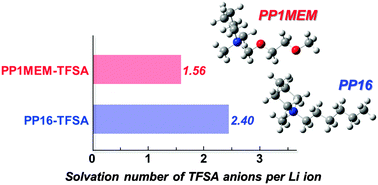
As the development of high energy-density Li-ion batteries moves ahead, ensuring safety of the batteries has become increasingly important. Among the unique physicochemical properties of ionic liquids, thermal stability can be one of the answers to the challenge. The use of ionic liquids, however, causes critical issues concerning the kinetics of Li-ion transfer at the electrode–electrolyte interface. In the present study, ionic liquids consisting of 1-((2-methoxyethoxy)methyl)-1-methylpiperidinium (PP1MEM) or 1-hexyl-1-methylpiperidinium (PP16) and bis(trifluoromethanesulfonyl)amide (TFSA) were applied to an electrolyte for Li-ion batteries, and we investigated the effect of cation structure on interfacial Li-ion transfer using Li4Ti5O12 as a model electrode by means of Raman spectroscopy and electrochemical impedance spectroscopy. It was found that the ether functional group in the PP1MEM cation has the meaningful function; the cation structure reduces the electrostatic interaction between the Li ion and TFSA anions in an ionic liquid electrolyte. The solvation number of the TFSA anion per Li ion consequently became smaller than that in PP16-TFSA, and the lower solvation number in PP1MEM-TFSA allowed the facile Li-ion diffusion in the electrolyte bulk rather than the interfacial Li-ion transfer and significantly improved the rate performance. The results offer the prospect of utilization of PP1MEM-TFSA as an electrolyte solvent. The knowledge obtained from this study contributes to the development of next-generation Li-ion batteries having both high energy density and high safety.


 Please wait while we load your content...
Please wait while we load your content...