The (0001) surfaces of α-Fe2O3 nanocrystals are preferentially activated for water oxidation by Ni doping†
Abstract
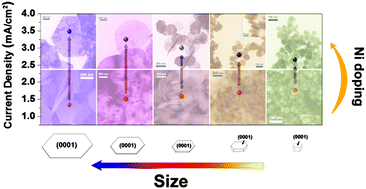
Photoelectrochemical water oxidation on hematite has been extensively studied, yet the relationship between the various facets exposed, heteroatom doping, and associated electrocatalytic activity has not been adequately explored. Here, hematite nanocrystals were synthesized with continuous tuning of the aspect-ratio and fine control of the surface area ratio of the (0001) facet with respect to other surfaces. The samples were doped with nickel, which was confirmed using the combined results of HRTEM, SEM, XRD, Raman, BET, and XPS measurements. The surface area ratio of the hexagonal (0001) surface with respect to all surfaces was tuned from 98% to 30%. Ni doping was accomplished by diffusion of Ni clusters into the subsurface region, which forms a uniformly doped NixFe2−xO3 surface overlayer that improves the electrocatalytic activity of water oxidation. These results are discussed in the context of a theoretical prediction and subsequent surface science validation that Ni doping facilitates the water oxidation reaction on hematite (0001) surfaces. Electrochemical testing of water oxidation catalysis was carried out on doped and shape-controlled hematite nanocrystals. The enhancement of water oxidation activity by Ni-doping increased as the surface area ratio of the (0001) facet of hematite nanocrystals increased, consistent with the theoretical predictions and surface science studies.

 Please wait while we load your content...
Please wait while we load your content...