Effect of electron-donating substituent groups on aromatic ring on photoluminescence properties of complexes of benzoic acid-functionalized polysulfone with Eu(iii) ions
Abstract
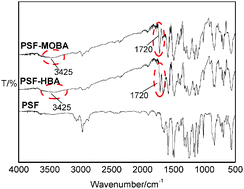
By molecular design and via polymer reactions, methoxybenzoic acid (MOBA) and hydroxybenzoic acid (HBA) were bonded onto the side chains of polysulfone (PSF) for preparing two benzoic acid-functionalized PSFs, PSF-MOBA and PSF-HBA, respectively. Based on full characterization of their structures, the two macromolecule ligands were made to coordinate to Eu3+ ions, and two binary polymer-rare earth complexes, PSF-(MOBA)3–Eu(III) and PSF-(HBA)3–Eu(III), were obtained. At the same time, using phenanthroline (Phen) as a second small-molecule ligand, the corresponding two ternary complexes, PSF-(MOBA)3–Eu(III)-Phen1 and PSF-(HBA)3–Eu(III)-Phen1, were also prepared. The photo physical behaviors of these complexes were examined in depth, and the luminescent properties of these prepared polymer-rare earth complexes were mainly investigated. The experimental results show that the two electron-donating substituent groups on the aromatic ring of the bonded benzoic acid significantly affect the luminescence properties of these complexes of benzoic acid-functionalized PSF and Eu(III) ions, and they can effectively strengthen the fluorescence emission intensities of the complexes. The possible reason is that through the p–π conjugative effect, the two electron-donating substituent groups can remarkably decline the triplet state energy levels of the bonded ligand MOBA and HBA, and strengthen the matching degree of energy between the triplet state energy level of the ligand and the resonant energy level of Eu(III) ions, resulting in the enhancement of fluorescence emission intensities of the complexes. Besides, the fluorescence emissions of the binary complexes are stronger than those of the corresponding ternary complexes because of the synergistic coordination effect of Phen with the macromolecular ligand.

 Please wait while we load your content...
Please wait while we load your content...