The role of alkoxy radicals in the heterogeneous reaction of two structural isomers of dimethylsuccinic acid†
Abstract
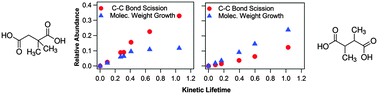
A key challenge in understanding the transformation chemistry of organic aerosols is to quantify how changes in molecular structure alter heterogeneous reaction mechanisms. Here we use two model systems to investigate how the relative locations of branched methyl groups control the heterogeneous reaction of OH with two isomers of dimethylsuccinic acid (C6H10O4). 2,2-Dimethylsuccinic acid (2,2-DMSA) and 2,3-dimethylsuccinic acid (2,3-DMSA) differ only in the location of the two branched methyl groups, thus enabling a closer inspection of how the distribution of carbon reaction sites impacts the chemical evolution of the aerosol. The heterogeneous reaction of OH with 2,3-DMSA (reactive OH uptake coefficient, γ = 0.99 ± 0.16) is found to be ∼2 times faster than that of 2,2-DMSA (γ = 0.41 ± 0.07), which is attributed to the larger stability of the tertiary alkyl radical produced by the initial OH abstraction reaction. While changes in the average aerosol oxidation state (OSC) and the carbon number (NC) are similar for both isomers upon reaction, significant differences are observed in the underlying molecular distribution of reaction products. The reaction of OH with the 2,3-DMSA isomer produces two major reaction products: a product containing a new alcohol functional group (C6H10O5) formed by intermolecular hydrogen abstraction and a C5 compound formed via carbon–carbon (C–C) bond scission. Both of these reaction products are explained by the formation and subsequent reaction of a tertiary alkoxy radical. In contrast, the OH reaction with the 2,2-DMSA isomer forms four dominant reaction products, the majority of which are C5 scission products. The difference in the quantity of C–C bond scission products for these two isomers is unexpected since decomposition is assumed to be favored for the isomer with the most tertiary carbon sites (i.e. 2,3-DMSA). For both isomers, there is a much larger abundance of C6 alcohol relative to C6 ketone products, which suggests that the presence of the two branched methyl groups favors alkoxy formation from peroxy radical self-reactions. These results reveal how the isomeric structure ultimately controls the overall competition between functionalization and fragmentation in these model systems.

 Please wait while we load your content...
Please wait while we load your content...