Mechanism for formation of atmospheric Cl atom precursors in the reaction of dinitrogen oxides with HCl/Cl− on aqueous films†
Abstract
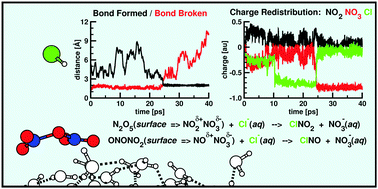
Nitryl chloride (ClNO2) and nitrosyl chloride (ClNO) are potential sources of highly reactive atmospheric chlorine atoms, hence of much interest, but their formation pathways are unknown. This work predicts production of these nitrogen oxychlorides from ab initio molecular dynamics (AIMD) simulations of N2O5 or an NO2 dimer on the surface of a thin film of water which is struck by gaseous HCl. Both of these heterogeneous reactions proceed at the liquid/vapor interface by an SN2 mechanism where the nucleophile is chloride ion formed from the ionization of HCl on the aqueous surface. The film of water enhances the otherwise very slow gas phase reaction to occur by (1) stabilizing and localizing the adsorbed N2O5 or NO2 dimer so it is physically accessible for reaction, (2) ionizing the impinging HCl, and (3) activating the adsorbed oxide for nucleophilic attack by chloride. Though both nitrogen oxychloride products are produced by SN2 reactions, the N2O5 mechanism is unusual in that the electrophilic N atom to be attacked oscillates between the two normally equivalent NO2 groups. Chloride ion is found to react with N2O5 less efficiently than with N2O4. The simulations provide an explanation for this. These substitution/elimination mechanisms are new for NOx/y chemistry on thin water films and cannot be derived from small cluster models.

 Please wait while we load your content...
Please wait while we load your content...