Modulating the interaction between gold and TiO2 nanowires for enhanced solar driven photoelectrocatalytic hydrogen generation†
Abstract
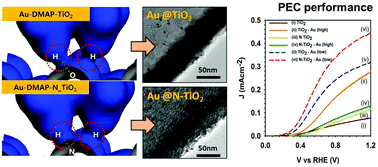
The interaction strength of Au nanoparticles with pristine and nitrogen doped TiO2 nanowire surfaces was analysed using density functional theory and their significance in enhancing the solar driven photoelectrocatalytic properties was elucidated. In this article, we prepared 4-dimethylaminopyridine capped Au nanoparticle decorated TiO2 nanowire systems. The density functional theory calculations show {101} facets of TiO2 as the preferred phase for dimethylaminopyridine–Au nanoparticles anchoring with a binding energy of −8.282 kcal mol−1. Besides, the interaction strength of Au nanoparticles was enhanced nearly four-fold (−35.559 kcal mol−1) at {101} facets via nitrogen doping, which indeed amplified the Au nanoparticle density on nitrided TiO2. The Au coated nitrogen doped TiO2 (N–TiO2–Au) hybrid electrodes show higher absorbance owing to the light scattering effect of Au nanoparticles. In addition, N–TiO2–Au hybrid electrodes block the charge leakage from the electrode to the electrolyte and thus reduce the charge recombination at the electrode/electrolyte interface. Despite the beneficial band narrowing effect of nitrogen in TiO2 on the electrochemical and visible light activity in N–TiO2–Au hybrid electrodes, it results in low photocurrent generation at higher Au NP loading (3.4 × 10−7 M) due to light blocking the N–TiO2 surface. Strikingly, even with a ten-fold lower Au NP loading (0.34 × 10−7 M), the synergistic effects of nitrogen doping and Au NPs on the N–TiO2–Au hybrid system yield high photocurrent compared to TiO2 and TiO2–Au electrodes. As a result, the N–TiO2–Au electrode produces nearly 270 μmol h−1 cm−2 hydrogen, which is nearly two-fold higher than the pristine TiO2 counterpart. The implications of these findings for the design of efficient hybrid photoelectrocatalytic electrodes are discussed.

 Please wait while we load your content...
Please wait while we load your content...