Excited state interactions between the chiral Au38L24 cluster and covalently attached porphyrin†
Abstract
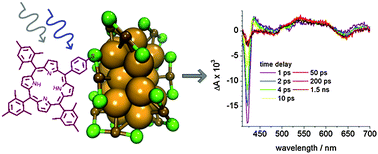
A protected S-acetylthio porphyrin was synthesized and attached to the Au38(2-phenylethanethiolate)24 cluster in a ligand exchange reaction. Chiral high performance liquid chromatography of the functionalized cluster yielded enantiomeric pairs of clusters probably differing in the binding site of the porphyrin. As proven by circular dichroism, the chirality was maintained. Exciton coupling between the cluster and the chromophore is observed. Zinc can be incorporated into the porphyrin attached to the cluster, as evidenced by absorption and fluorescence spectroscopy, however, the reaction is slow. Quenching of the chromophore fluorescence is observed, which can be explained by energy transfer from the porphyrin to the cluster. Transient absorption spectra of Au38(2-phenylethanethiolate)24 and the functionalized cluster probe the bleach of the gold cluster due to ground state absorption and the characteristic excited state absorption signals. Zinc incorporation does not have a pronounced effect on the photophysical behaviour. Decay times are typical for the molecular behaviour of small monolayer protected gold clusters.

 Please wait while we load your content...
Please wait while we load your content...