Experimental determination of the partitioning coefficient of β-pinene oxidation products in SOAs
Abstract
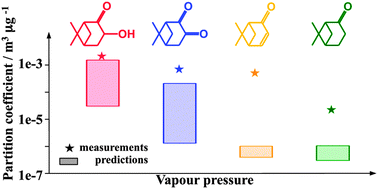
The composition of secondary organic aerosols (SOAs) formed by β-pinene ozonolysis was experimentally investigated in the Juelich aerosol chamber. Partitioning of oxidation products between gas and particles was measured through concurrent concentration measurements in both phases. Partitioning coefficients (Kp) of 2.23 × 10−5 ± 3.20 × 10−6 m3 μg−1 for nopinone, 4.86 × 10−4 ± 1.80 × 10−4 m3 μg−1 for apoverbenone, 6.84 × 10−4 ± 1.52 × 10−4 m3 μg−1 for oxonopinone and 2.00 × 10−3 ± 1.13 × 10−3 m3 μg−1 for hydroxynopinone were derived, showing higher values for more oxygenated species. The observed Kp values were compared with values predicted using two different semi-empirical approaches. Both methods led to an underestimation of the partitioning coefficients with systematic differences between the methods. Assuming that the deviation between the experiment and the model is due to non-ideality of the mixed solution in particles, activity coefficients of 4.82 × 10−2 for nopinone, 2.17 × 10−3 for apoverbenone, 3.09 × 10−1 for oxonopinone and 7.74 × 10−1 for hydroxynopinone would result using the vapour pressure estimation technique that leads to higher Kp. We discuss that such large non-ideality for nopinone could arise due to particle phase processes lowering the effective nopinone vapour pressure such as diol- or dimer formation. The observed high partitioning coefficients compared to modelled results imply an underestimation of SOA mass by applying equilibrium conditions.

 Please wait while we load your content...
Please wait while we load your content...