Structural morphologies of high-pressure polymorphs of strontium hydrides†
Abstract
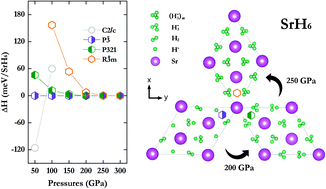
It is now known that the structure and properties of a material can be significantly altered under extreme compression. In this work, a structural search was performed to investigate the phase stabilities and structures of SrH2n (n = 1–5) in the pressure range of 50–300 GPa. The high-pressure polymorphs reveal a variety of hydrogen structural units ranging from monatomic hydride to linear and bent H3 and spiral polymer chains. A novel graphene like H-layer structure was found to exist in SrH10 at 300 GPa. The structural diversity in the predicted high pressure structures provides an opportunity for an in-depth analysis of the chemical bonding in the high pressure polyhydrides. It is shown from theoretical calculations that the electronegativity of molecular hydrogen is similar to that of group 13 and 14 elements. This resulted in electrons being transferred from Sr to the hydrogen molecules. Thus, a consideration of the number of valence electrons available from Sr that can be shared among the H2 serves as a useful guide to rationalize the structures of the H-moieties. An alternative description of the high pressure structures differing from a previous study is presented here.

 Please wait while we load your content...
Please wait while we load your content...