Properties of transition metal substituted zinc sulfide hexamers and dodecamers
Abstract
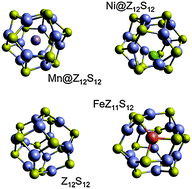
Density functional theory was used to study the structural and electronic properties of endohedrally- and substitutionally-doped Zn6S6 and Zn12S12 clusters with first-row transition metal atoms. Generally, the lowest energy electronic state of the cluster is that with the maximum multiplicity (Ti and Cr are exceptions). Substitutionally-doped clusters have greater binding energies (per atom) for both cluster sizes, providing an indication that similar doping will be preferred in the bulk material as well. The results are relevant to thin films of doped ZnS in which cluster formation is likely.

 Please wait while we load your content...
Please wait while we load your content...