Hydrogels formed from Fmoc amino acids†
Abstract
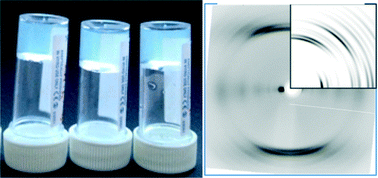
A number of Fmoc amino acids can be effective low molecular weight hydrogelators. The type of gel formed depends on the amino acid used and, in the case of FmocF, the final pH of the system. The single crystal structure of two of the gelators (FmocF and FmocY) have been determined and the data compared to the fibre X-ray diffraction data. FmocF, which forms metastable gels, crystallises easily and the data for the fibre phase and crystal phase are relatively similar. For FmocF, the fibre axis in b is consistent with the hydrogen bonding repeat distances and the diffraction pattern calculated from the single crystal structure is a good match with the experimental fibre X-ray diffraction data. On the other hand, there are significant differences between the crystalline phase determined and the fibre phase for FmocY. The packing of FmocY within the crystal structure is created by interactions between the planar Fmoc groups, whilst it is clear that hydrogen bonding drives the self-assembly into fibrillar structures within the gels. This shows that understanding the packing in gel phase by analogy to isolated crystal structures has the potential to lead to erroneous conclusions.
- This article is part of the themed collection: Supramolecular Gels in Crystal Engineering

 Please wait while we load your content...
Please wait while we load your content...