Structural diversity of luminescent lanthanide metal–organic frameworks based on a V-shaped ligand†
Abstract
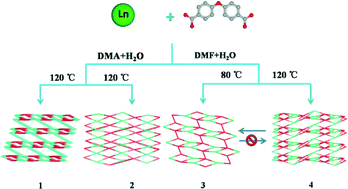
Four types of luminescent lanthanide metal–organic frameworks (Ln-MOFs) with the formulas [Ln2(OBA)3(DMA)2(H2O)2]n [1, Ln = Eu (1-Eu), Tb (1-Tb)], {[Ln2(OBA)3(H2O)4]·2H2O}n [2, Ln = Eu (2-Eu), Tb (2-Tb)], {[Ln(OBA)(HOBA)(H2O)2]·3DMF}n [3, Ln = Eu (3-Eu), Tb (3-Tb)], and [Ln2(OBA)3(DMF)(H2O)2]n [4, Ln = Eu (4-Eu), Tb (4-Tb)] (H2OBA = 4,4′-oxybis(benzoate) acid, DMA = N,N-dimethylacetamide, DMF = N,N-dimethylformamide) have been solvothermally synthesized based on a V-shaped ligand H2OBA under different conditions. The structural features of the four types of Ln-MOFs are as follows: 1 shows a 3D framework in which the dinuclear SBUs are further cross-linked by OBA2− ligands. Both 2 and 3 exhibit a 2D network constructed by 1D chains. 4 features a 2D network, in which 1D chains and dinuclear SBUs arranged alternately through the OBA2− ligands. 4 can be transformed to 3 at room temperature in mother solution. The experimental results reveal that solvent and temperature play important roles in constructing coordination polymers. All the aforementioned Ln-MOFs are fully characterized by elemental analysis, infrared spectroscopy, and thermogravimetric analysis. The luminescence properties of these Ln-MOFs have been studied, showing emission characteristic for inorganic species at room temperature.

 Please wait while we load your content...
Please wait while we load your content...