Allosteric stabilization of the amyloid-β peptide hairpin by the fluctuating N-terminal†
Abstract
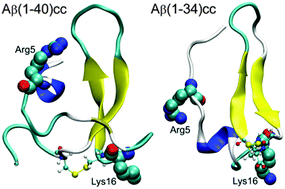
Immobilized ions modulate nearby hydrophobic interactions and influence molecular recognition and self-assembly. We simulated disulfide bond-locked double mutants (L17C/L34C) and observed allosteric modulation of the peptide's intra-molecular interactions by the N-terminal tail. We revealed that the non-contacting charged N-terminal residues help the transfer of entropy to the surrounding solvation shell and stabilizing β-hairpin.

 Please wait while we load your content...
Please wait while we load your content...