Dinuclear planar chiral ferrocenyl gold(i) & gold(ii) complexes†
Abstract
Oxidation of Au(I) in the presence of Fe(II) allowed for the synthesis of unique dinuclear ferrocenyl Au(II) complexes via the first reported enantiopure planar chiral ferrocenyl Au(I) complex. (Spectro)electrochemical studies show that oxidation at Fe(II) is favoured, but DFT studies suggest that the energy differences for oxidation of one or the other metal should be quite small.
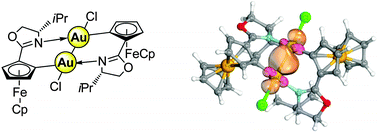

 Please wait while we load your content...
Please wait while we load your content...