An alternative synthesis of the breast cancer drug fulvestrant (Faslodex®): catalyst control over C–C bond formation†
Abstract
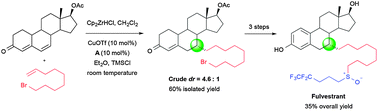
Fulvestrant (Faslodex®) was synthesized in four steps (35% overall yield) from 6-dehydronandrolone acetate. Catalyst controlled, room temperature, diastereoselective 1,6-addition of the zirconocene derived from commercially available 9-bromonon-1-ene was used in the key C–C bond forming step.

 Please wait while we load your content...
Please wait while we load your content...