Asymmetric cyclopropanation of conjugated cyanosulfones using a novel cupreine organocatalyst: rapid access to δ3-amino acids†
Abstract
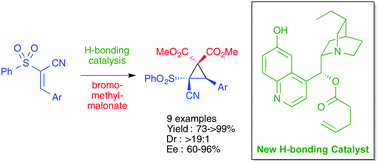
An organocatalytic asymmetric synthesis of a novel, highly functionalised cyclopropane system furnished with versatile substituents and containing a quaternary centre is described. The process utilises a new bifunctional catalyst based on the cinchona alkaloid framework and the products made using this catalyst were obtained as single diastereoisomers, with very high enantioselectivities (up to 96% ee). We have also demonstrated that these resulting cyclopropanes are very useful synthetic intermediates to interesting products, such as the difficult to access δ3-amino acids.

 Please wait while we load your content...
Please wait while we load your content...