A recyclable CO surrogate in regioselective alkoxycarbonylation of alkenes: indirect use of carbon dioxide†
Abstract
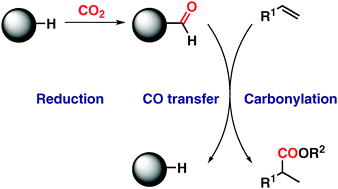
Herein, we report a Pd-catalysed alkoxycarbonylation of alkenes based on the use of a recyclable CO2 reduction product, the crystalline and air-stable N-formylsaccharin, as a CO surrogate. The carbonylation proceeds under ambient conditions in an exceptionally complementary regioselective fashion yielding the desired branched products from styrene derivatives and valuable linear esters from alkyl-substituted alkenes.

 Please wait while we load your content...
Please wait while we load your content...