Site-specific indolation of proline-based peptides via copper(ii)-catalyzed oxidative coupling of tertiary amine N-oxides†
Abstract
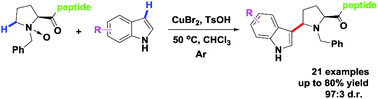
The first site-specific and purely chemical method for modifying proline-based peptides was developed via a convenient, copper-catalyzed oxidative coupling of tertiary amine N-oxides with indoles. This novel approach features high regioselectivity and diastereoselectivity, mild conditions, and compatibility with various functional groups. In addition, a simplified process was realized in one pot and two steps via in situ oxidative coupling of tertiary amine and indoles.

 Please wait while we load your content...
Please wait while we load your content...