Facile synthesis of 1-trifluoromethylalkenes via the decarboxylation of α-trifluoromethyl-β-lactones†
Abstract
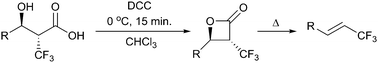
DCC-mediated cyclodehydration of α-trifluoromethyl-β-hydroxy acids provides α-trifluoromethylated β-lactone intermediates, without loss of stereoselectivity. These lactones undergo facile decarboxylation providing a simple route to obtain both alkyl and aryl trifluoromethylated alkenes in excellent yields and stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...