A biomimetic phenol substituent effect on the reaction of a dimethylplatinum(ii) complex with oxygen: proton coupled electron transfer and multiple proton relay†
Abstract
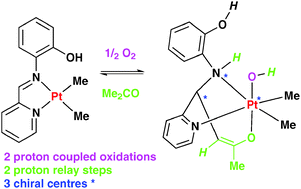
The complex [PtMe2({κ2-N,N-RN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-2-C5H4N})] reacts with oxygen in acetone solution to give the platinum(IV) complex [Pt(OH)Me2{κ3-N,N,O-RNH–CH(2-C5H4N)(CH
CH-2-C5H4N})] reacts with oxygen in acetone solution to give the platinum(IV) complex [Pt(OH)Me2{κ3-N,N,O-RNH–CH(2-C5H4N)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMeO)}], when R = 2-C6H4OH, but not when R = Ph. It has been suggested that the phenol substituent plays two key biomimetic roles; firstly, in proton coupled electron transfer reactions in the activation of oxygen and hydroperoxide groups and, secondly, in proton relay from a methyl group of the coordinated acetone.
CMeO)}], when R = 2-C6H4OH, but not when R = Ph. It has been suggested that the phenol substituent plays two key biomimetic roles; firstly, in proton coupled electron transfer reactions in the activation of oxygen and hydroperoxide groups and, secondly, in proton relay from a methyl group of the coordinated acetone.

 Please wait while we load your content...
Please wait while we load your content...