The first case of actinide triple helices: pH-dependent structural evolution and kinetically-controlled transformation of two supramolecular conformational isomers†
Abstract
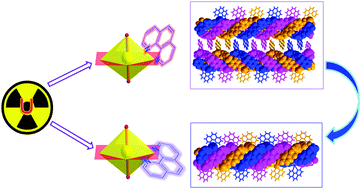
The first actinide triple helices, including two supramolecular conformational isomers of uranium(VI), have been synthesized with the aid of a flexible V-shaped ligand and a rigid aromatic base. The isomers exhibit an intriguing pH-dependent structural evolution and a kinetically-controlled transformation via a novel conformational rearrangement of the organic base.

 Please wait while we load your content...
Please wait while we load your content...