Acylative Suzuki coupling of amides: acyl-nitrogen activation via synergy of independently modifiable activating groups†
Abstract
A highly efficient palladium-catalyzed acylative cross-coupling of carboxylic amides with arylboronic acids has been achieved via synergistic activation of the Cacyl–N bond by independently modifiable activating groups. Coupling of amides features not only good functional group tolerance but also modifiable reactivities to overcome steric hindrance.
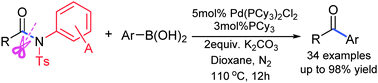

 Please wait while we load your content...
Please wait while we load your content...