Copper-catalyzed ortho-halogenation of arenes and heteroarenes directed by a removable auxiliary†
Abstract
Copper-catalyzed ortho-halogenation of C(sp2)–H bonds directed by a PIP directing group with NXS (X = Cl, Br, I) has been developed. The reaction is scalable and tolerates a broad range of functional groups and heteroarenes, providing an efficient access to halogenated arenes and heteroarenes.
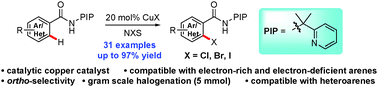

 Please wait while we load your content...
Please wait while we load your content...