PNGase F-mediated incorporation of 18O into glycans for relative glycan quantitation†
Abstract
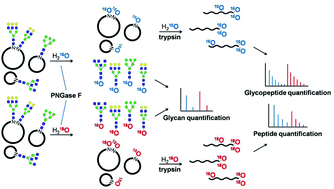
PNGase F-catalyzed glycosylation site 18O-labeling is a widely used method for glycoprotein quantitation owing to its efficiency and simplicity. However, PNGase F-catalyzed glycan 18O-labeling, which offers advantages for glycomics, has not yet been developed. In this study, PNGase F-mediated incorporation of 18O into glycans during the N-glycan release from glycoproteins by PNGase F was finally realized, named as PCGOL (PNGase F-catalyzed glycan 18O-labeling), which offers a potential strategy for relative glycan quantitation. This new method showed good linearity and high reproducibility within at least 2 orders of magnitude in the dynamic range. Furthermore, PCGOL combined with our previously developed TOSIL method (tandem 18O stable isotope labeling for N-glycoproteome quantitation) can be used for comprehensive N-glycosylation quantification, achieving simultaneous quantification of glycans, glycopeptides and glycoproteins in a single workflow, which was also used to analyze glycosylation changes in immunoglobulin G (IgG) associated with hepatocellular carcinoma in the present work.

 Please wait while we load your content...
Please wait while we load your content...