Topochemical conversion of a dense metal–organic framework from a crystalline insulator to an amorphous semiconductor†
Abstract
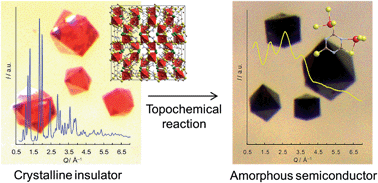
The topochemical conversion of a dense, insulating metal–organic framework (MOF) into a semiconducting amorphous MOF is described. Treatment of single crystals of copper(I) chloride trithiocyanurate, CuICl(ttcH3) (ttcH3 = trithiocyanuric acid), 1, in aqueous ammonia solution yields monoliths of amorphous CuI1.8(ttc)0.6(ttcH3)0.4, 3. The treatment changes the transparent orange crystals of 1 into shiny black monoliths of 3 with retention of morphology, and moreover increases the electrical conductivity from insulating to semiconducting (conductivity of 3 ranges from 4.2 × 10−11 S cm−1 at 20 °C to 7.6 × 10−9 S cm−1 at 140 °C; activation energy = 0.59 eV; optical band gap = 0.6 eV). The structure and properties of the amorphous conductor are fully characterized by AC impedance spectroscopy, X-ray photoelectron spectroscopy, X-ray pair distribution function analysis, infrared spectroscopy, diffuse reflectance spectroscopy, electron spin resonance spectroscopy, elemental analysis, thermogravimetric analysis, and theoretical calculations.
- This article is part of the themed collection: In celebration of Tony Cheetham’s 70th birthday

 Please wait while we load your content...
Please wait while we load your content...