Enantioselective reductive multicomponent coupling reactions between isatins and aldehydes†
Abstract
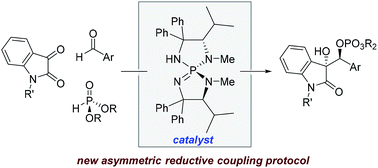
A metal-free stereoselective reductive coupling reaction between isatins and aldehydes is reported. The reaction relies on commercial diethyl phosphite (∼€70 kg−1) as the stoichiometric reductant. Base-catalyzed Pudovik addition and phosphonate/phosphate rearrangement achieved polarity inversion on the isatin, and the derived carbanions were trapped by aldehydes with subsequent dialkoxyphosphinyl migration. Chiral iminophosphoranes were used as basic catalysts to achieve high diastereo- and enantioselectivities with excellent yields.

 Please wait while we load your content...
Please wait while we load your content...