Dielectric-dependent electron transfer behaviour of cobalt hexacyanides in a solid solution of sodium chloride
Abstract
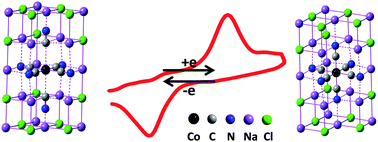
Here we emphasise the importance of the dielectric environment on the electron transfer behavior in interfacial electrochemical systems. Through doping cobalt hexacyanide (Co(CN)63−) into single microcrystals of sodium chloride (NaCl), for the first time, we obtained the direct electrochemical behavior of Co(CN)63− which is hardly ever obtained in either aqueous or conventional nonaqueous solutions. DFT calculations elucidate that, as the Co(CN)63− anions occupy the lattice units of NaCl65− in the NaCl microcrystal, the redox energy barrier of Co(CN)63−/4− is decreased dramatically due to the low dielectric constant of NaCl. Meanwhile, the low-spin Co(CN)64− anions are stabilized in the lattices of the NaCl microcrystal. The results also show that the NaCl microcrystal is a potential solvent for solid-state electrochemistry at ambient temperature.

 Please wait while we load your content...
Please wait while we load your content...