Ion structure controls ionic liquid near-surface and interfacial nanostructure†
Abstract
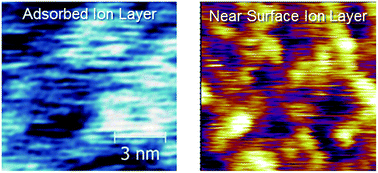
A unique, but unifying, feature of ionic liquids (ILs) is that they are nanostructured on the length scale of the ions; in many ILs well-defined polar and apolar domains exist and may percolate through the liquid. Near a surface the isotropic symmetry of the bulk structure is broken, resulting in different nanostructures which, until now, have only been studied indirectly. In this paper, in situ amplitude modulated atomic force microscopy (AM-AFM) has been used to resolve the 3-dimensional nanostructure of five protic ILs at and near the surface of mica. The surface and near surface structures are distinct and remarkably well-defined, but are very different from previously accepted descriptions. Interfacial nanostructure is strongly influenced by the registry between cations and the mica surface charge sites, whereas near surface nanostructure is sensitive to both cation and anion structure. Together these ILs reveal how interfacial nanostructure can be tuned through ion structure, informing “bottom-up” design and optimisation of ILs for diverse technologies including heterogeneous catalysis, lubrication, electrochemical processes, and nanofluids.

 Please wait while we load your content...
Please wait while we load your content...