Ligand functionalization as a deactivation pathway in a fac-Ir(ppy)3-mediated radical addition†
Abstract
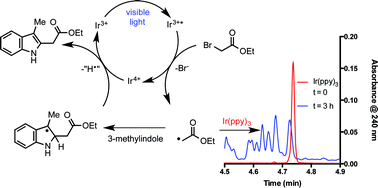
Knowledge of the kinetic behavior of catalysts under synthetically relevant conditions is vital for the efficient use of compounds that mediate important transformations regardless of their composition or driving force. In particular, these data are of great importance to add perspective to the growing number of applications of photoactive transition metal complexes. Here we present kinetic, synthetic, and spectroscopic evidence of the mechanistic behavior of fac-Ir(ppy)3 in a visible light-mediated radical addition to 3-methylindole, demonstrating the instability of fac-Ir(ppy)3 under these conditions. During the reaction, rapid in situ functionalization of the photocatalyst occurs, eventually leading to deactivation. These findings demonstrate a conceivable deactivation process for catalytic single electron reactions in the presence of radicophilic ligands. Attempts to inhibit photocatalyst deactivation through structural modification provide further insight into catalyst selection for a given system of interest.

 Please wait while we load your content...
Please wait while we load your content...