The application of chiroptical spectroscopy (circular dichroism) in quantifying binding events in lanthanide directed synthesis of chiral luminescent self-assembly structures†
Abstract
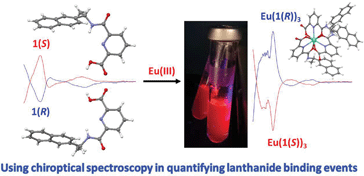
The binding of asymmetrical and optically pure tridentate ligands (L = 1(S) and 1(R)) containing one carboxylic group and 2-naphthyl as an antenna to lanthanide ions (M = La(III) and Eu(III)) was studied in CH3CN, showing the successive formation of M:L, M:L2 and M:L3 stoichiometric species in solution. The europium complexes EuL3 were also synthesised, structurally characterised and their photophysical properties probed in CH3OH and CH3CN. The changes in the chiroptical properties of both 1(S) and 1(R) were used (by circular dichroism (CD) spectroscopy) to monitor the formation of these chiral self-assemblies in solution. While circularly polarised luminescence (CPL) showed the formation of Eu(1(S))3 and Eu(1(R))3 as enantiomers, with high luminescence dissymmetry factors (glum), fitting the CD changes allowed for binding constants to be determined that were comparable to those seen in the analyses of absorbance and luminescence changes.

 Please wait while we load your content...
Please wait while we load your content...