Sandmeyer cyanation of arenediazonium tetrafluoroborate using acetonitrile as a cyanide source†
Abstract
Palladium-catalyzed cyanation of aryldiazonium tetrafluoroborate using acetonitrile as a non-metallic cyanide source was achieved in the presence of Ag2O under ambient air, eliminating the involvement of highly toxic CuCN used in the traditional Sandmeyer reaction, in which the CN group comes from metallic cyanides. The substrate scope and limitation of this protocol were investigated.
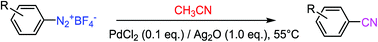

 Please wait while we load your content...
Please wait while we load your content...