Selective syntheses of leuconolam, leuconoxine, and mersicarpine alkaloids from a common intermediate through regiocontrolled cyclizations by Staudinger reactions†
Abstract
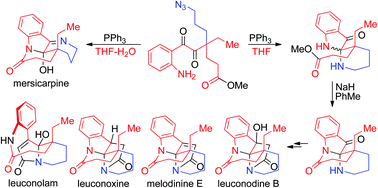
Selective syntheses of leuconolam, leuconoxine, and mersicarpine alkaloids bearing distinctive core structures were achieved through Staudinger reactions using a common intermediate. In the key cyclization step, water functioned like a switch to control which core structure to produce. The chemistry allowed for selective syntheses of the group of alkaloids from a simple intermediate through straightforward chemical operations.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2015

 Please wait while we load your content...
Please wait while we load your content...