Enantioselective Friedel–Crafts reaction of 4,7-dihydroindoles with β-CF3-β-disubstituted nitroalkenes†
Abstract
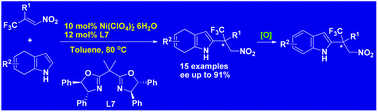
Using a Ni(ClO4)2–bisoxazoline complex as a catalyst, Friedel–Crafts alkylations of 4,7-dihydroindoles with β-CF3-β-disubstituted nitroalkenes were carried out with high enantioselectivities (up to 91%) to give alkylated dihydroindoles bearing trifluoromethylated all-carbon quaternary stereocenters in good yields. The corresponding chiral C2 alkylated indoles were obtained with complete preservation of enantiomeric purity by oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ).

 Please wait while we load your content...
Please wait while we load your content...