Fibrillar gels via the self-assembly of poly(l-glutamate)-based statistical copolymers†
Abstract
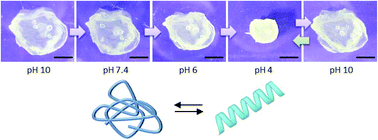
Polypeptides having secondary structures often undergo self-assembly which can extend over multiple length scales. Poly(γ-benzyl-L-glutamate) (PBLG), for example, folds into α-helices and forms physical organogels, whereas poly(L-glutamic acid) (PLGA at acidic pH) or poly(L-glutamate) (PLG at neutral/basic pH) do not form hydrogels. We explored the gelation of modified PBLG and investigated the deprotection of the carboxylic acid moieties in such gels to yield unique hydrogels. This was accomplished through photo-crosslinking gelation of poly(γ-benzyl-L-glutamate-co-allylglycine) statistical copolymers in toluene, tetrahydrofuran, and 1,4-dioxane. Unlike most polymer-based chemical gels, our gels were prepared from dilute solutions (<20 g L−1, i.e., <2% w/v) of low molar mass polymers. Despite such low concentrations and molar masses, our dioxane gels showed high mechanical stability and little shrinkage; remarkably, they also exhibited a porous fibrillar network. Deprotection of the carboxylic acid moieties in dioxane gels yielded pH responsive and highly absorbent PLGA/PLG-based hydrogels (swelling ratio of up to 87), while preserving the network structure, which is an unprecedented feature in the context of crosslinked PLGA gels. These outstanding properties are highly attractive for biomedical materials.

 Please wait while we load your content...
Please wait while we load your content...