An enantioselective organocatalyzed aza-Morita–Baylis–Hillman reaction of isatin-derived ketimines with acrolein†
Abstract
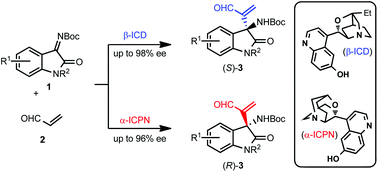
A highly enantioselective aza-Morita–Baylis–Hillman (aza-MBH) reaction of isatin-derived ketimines with acrolein was established using β-isocupreidine (β-ICD) or α-isocupreine (α-ICPN) as a chiral acid–base organocatalyst. The present protocol readily furnished (S) or (R)-aza-MBH adducts with a chiral tetrasubstituted carbon stereogenic center in up to 98% ee.

 Please wait while we load your content...
Please wait while we load your content...