PYRROC: the first functionalized cycloalkyne that facilitates isomer-free generation of organic molecules by SPAAC†
Abstract
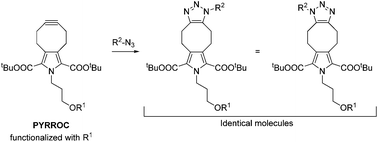
We present the concept, synthesis, and kinetic characterization of PYRROC as the first functionalized cycloalkyne which cannot form isomers in the reaction with azides. In aqueous buffer, PYRROC displays unprecedented rate accelerations in SPAAC of three to four orders of magnitude, leading to rate constants exceeding 400 M−1 s−1.

 Please wait while we load your content...
Please wait while we load your content...