Transition-metal-free C–H oxidative activation: persulfate-promoted selective benzylic mono- and difluorination†
Abstract
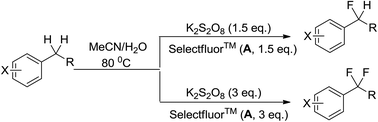
An operationally simple and selective method for the direct conversion of benzylic C–H to C–F to obtain mono- and difluoromethylated arenes using Selectfluor™ as a fluorine source is developed. Persulfate can be used to selectively activate benzylic hydrogen atoms toward C–F bond formation without the aid of transition metal catalysts.

 Please wait while we load your content...
Please wait while we load your content...