Regiodivergent Lewis base-promoted O- to C-carboxyl transfer of furanyl carbonates†
Abstract
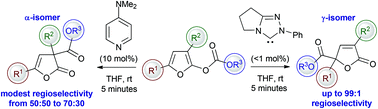
Triazolinylidenes promote γ-selective C-carboxylation (up to 99 : 1 regioselectivity) in the O- to C-carboxyl transfer of furanyl carbonates in contrast to DMAP that promotes preferential α-C-carboxylation with moderate regiocontrol (typically 60 : 40 regioselectivity). The generality of this process is described and a simple mechanistic and kinetic model postulated to account for the observed regioselectivity

 Please wait while we load your content...
Please wait while we load your content...