Shell decoration of hydrothermally obtained colloidal carbon spheres with base metal nanoparticles†
Abstract
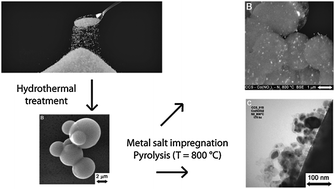
The preparation of base metal nanoparticles supported on the shell of colloidal carbon spheres (CCS) is reported. Hydrothermal treatment of a sucrose solution gave conglomerates of ca. 30 μm of CCS (diameter 2–8 μm), which consist of a hydrophobic core with a hydrophilic shell due to the presence of oxygen containing functional groups. The CCS were loaded by wet impregnation with various metal salts (copper, nickel, cobalt, iron). Subsequent pyrolysis under inert conditions at T = 800 °C led to the carbothermal reduction of the impregnated metal salts by the support material. The base metal nanoparticles (size ca. 35–70 nm) are supported on the circumference of the CCS in line with its core–shell structure. Moreover, in the case of nickel, cobalt and iron nanoparticles, all capable of forming metastable metal carbides, the carbonised shells are converted into nanostructures of graphitic carbon, viz., catalytic graphitisation occurs. The spheres were characterised by scanning- and transmission electron microscopy, X-ray diffraction, Raman spectroscopy, elemental analysis, infrared spectroscopy and thermogravimetric analysis.

 Please wait while we load your content...
Please wait while we load your content...