Synthesis and characterisation of novel aluminium and gallium precursors for chemical vapour deposition†
Abstract
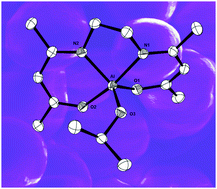
The β-ketoimine ligand [(Me)CN(H){iPr}–CHC(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] (L1H) and the bis(β-ketoimine) ligands [(CH2)2{N(H)C(Me)–CHC(R)
O] (L1H) and the bis(β-ketoimine) ligands [(CH2)2{N(H)C(Me)–CHC(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O}2] (L2H2, R = Me; L3H2, R = C6H5) linked by ethylene bridges have been used to form aluminium and gallium complexes: [Al(L1)Et2] (1), [Ga(L1)2Cl] (2), [AlL2(OiPr)] (3) and [GaL3Me] (4). The complexes were characterised by NMR spectroscopy, mass spectroscopy, and single crystal X-ray diffraction, with the exception of 1 which was isolated as an oil. Compounds 1–4 have been used for the first time as single source precursors for the deposition of Al2O3 (1, 3) and Ga2O3 (2, 4) respectively. Thin films were deposited via aerosol assisted (AA)CVD with toluene as the solvent.
O}2] (L2H2, R = Me; L3H2, R = C6H5) linked by ethylene bridges have been used to form aluminium and gallium complexes: [Al(L1)Et2] (1), [Ga(L1)2Cl] (2), [AlL2(OiPr)] (3) and [GaL3Me] (4). The complexes were characterised by NMR spectroscopy, mass spectroscopy, and single crystal X-ray diffraction, with the exception of 1 which was isolated as an oil. Compounds 1–4 have been used for the first time as single source precursors for the deposition of Al2O3 (1, 3) and Ga2O3 (2, 4) respectively. Thin films were deposited via aerosol assisted (AA)CVD with toluene as the solvent.

 Please wait while we load your content...
Please wait while we load your content...