Effect of molecular characteristics on the formation of nitrosamines during chlor(am)ination of phenylurea herbicides
Abstract
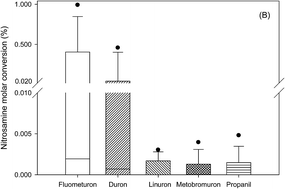
The objective of this study was to investigate the formation of different nitrosamines during chlorination or chloramination (chlor(am)ination) of five phenylurea herbicides (fluometuron, diuron, linuron, metobromuron, and propanil), with the effects of disinfection approaches, additional inorganic nitrogen, and reaction pH being studied. By analyzing six nitrosamines, N-nitrosodimethylamine (NDMA) and N-nitrosopyrrolidine (NPYR) formation was observed. The dimethylamine functional group was the key to determining whether a particular phenylurea herbicide was an important nitrosamine precursor, as the NDMA conversion ratio was much higher. Chlorination with ammonium or dichloramination enhanced the NDMA formation. NPYR formation from the herbicides that did not form NDMA was detected and was more vigorous during dichloramination or in the presence of either ammonium or nitrite. The NPYR formation was possibly related to the aniline molecular fragment from the phenylurea herbicides. Both NDMA and NPYR formation were higher at pH 8. Overall, the maximum nitrosamine conversions decreased in the order: fluometuron > diuron > propanil > metobromuron > linuron (up to 0.99%, 0.46%, 0.005%, 0.004%, and 0.003% molar conversion rates, respectively) during chlorination or chloramination and dichloramine > free chlorine > monochloramine (up to 0.99%, 0.41%, and 0.005% molar conversion rates, respectively) for given herbicide, chlorine, and nitrogen doses. Applying the results of this study, phenylurea herbicide concentrations ranging from several to tens of μg L−1 will yield a NDMA concentration in drinking water above the level for a theoretical 10−6 lifetime cancer risk. NPYR formation will increase the risk of these phenylurea herbicide concentrations to downstream water users. The true adverse environmental impacts of these phenylurea herbicides are important to emphasize given their high loadings as non-point source pollutants and their typical environmental scenarios (e.g., at neutral pH or with the co-occurrence of inorganic nitrogen), likely resulting in more efficient nitrosamine formation.

 Please wait while we load your content...
Please wait while we load your content...