Ternary transition-metal fluoride precursors for the fluorolytic sol–gel route: new insights into speciation and decomposition†
Abstract
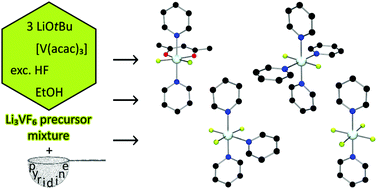
The nanoscaled ternary transition-metal fluorides Li3MF6 (M = V, Fe, Mn) and Li2NiF4 are promising candidates for cathode materials in high-voltage lithium-ion batteries. The fluorolytic route to these compounds relies on thermal decomposition of a hitherto uncharacterised precursor mixture produced from acetylacetonates and hydrofluoric acid. By addition of pyridine, different cationic, electroneutral and anionic complexes containing the motifs [MFn](3−n)+ (n = 0–4) have been trapped and characterised by single-crystal X-ray diffraction and IR spectroscopy. Based on the results, a model of successive and incomplete fluorination is proposed for the speciation and formation of the precursor. The decomposition of the latter has been monitored via thermogravimetry (TG) and differential scanning calorimetry (DSC).

 Please wait while we load your content...
Please wait while we load your content...