The synthesis, structure, topology and catalytic application of a novel cubane-based copper(ii) metal–organic framework derived from a flexible amido tripodal acid†
Abstract
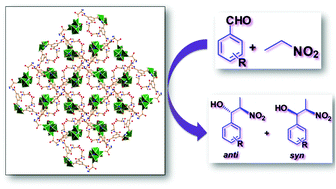
A novel chiral metal–organic framework, [Cu4(HL)2(H2O)4(MeO)4]n (1), has been successfully synthesized from a tripodal flexible ligand (2S,2′S,2′′S)-2,2′,2′′-(benzenetricarbonyltris(azanediyl))tripropanoic acid (H3L). Compound 1 was characterized by IR and X-ray powder diffraction analysis. The structure was determined by X-ray single crystal diffraction analysis revealing that 1 possesses a 3D network, featuring a tetranuclear cubane-type secondary building block [Cu4(MeO)4]4+, formed via the connection of four metal ions to four methoxide ions. These secondary building blocks are linked by four different HL2− ligands to construct a porous three dimensional framework of the dia topology with one-dimensional channels. Compound 1 also acts as a heterogeneous catalyst for the diastereoselective nitroaldol (Henry) reaction, providing high yields (up to 91%) and good diastereoselectivities under ambient conditions. This catalyst can be recycled without significant loss of activity.

 Please wait while we load your content...
Please wait while we load your content...