Sustainable production of dimethyl adipate by non-heme iron(iii) catalysed oxidative cleavage of catechol†
Abstract
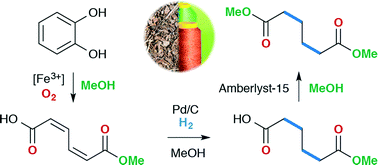
Adipic acid and its esters are important bulk chemicals whose principal use is in the production of the nylon-6,6 polymer. There is considerable interest in finding novel green routes from sustainable feedstocks towards these important intermediates. Herein, we describe the catalytic oxidative cleavage of catechol to muconic acids using a catalyst prepared in situ from iron(III) nitrate, tris(2-pyridylmethyl)amine and ammonium acetate. An investigation of catalyst loading, temperature and oxygen pressure, allowed a turnover frequency of 120 h−1 to be obtained. The subsequent hydrogenation and transesterification of the obtained muconic acid products were shown to proceed well over commercially available supported catalysts. After vacuum distillation, dimethyl adipate could be isolated in 62% yield from catechol, thus demonstrating a green and sustainable route to this important bulk chemical.

 Please wait while we load your content...
Please wait while we load your content...